Get your eLogBooks for FREE from Atachi Systems
Cheaper Manufacturing costs are not a competitive advantage for Indian Pharma Manufacturing companies
The industry needs to start the Pharma 4.0 Journey, and Why elogbooks is the first inexpensive step. Atachi systems are committed to helping you with the Pharam4.0 journey with free eLogbooks.
In this context, Atachi Systems will share the importance of eLogbooks for pharmaceutical manufacturing. If you look at the Indian pharma industry, many pharma manufacturing companies still use paper-based logbooks and records. These companies face a lot of challenges and compliance issues in the paper-based logbooks. Even though they record all the details about equipment cleaning, calibration, maintenance schedules, packing, weighing, and dispensing in a paper-based logbook; It’s prone to compliance issues, human errors, misplacement, and loss of productivity. They also need to validate the logs manually according to SOP procedures and operational requirements. It is quite a challenging and daunting task. What is draining the value for Pharma manufacturing companies is not realizing the value associated with capturing the information and generating its insights. It means the industry has been developing the logbooks per SOP’s only for the sake of auditors and CGMP Practices; Even then, many Pharma manufacturing companies are getting FDA warning letters because of cleanliness, data integrity, calibration, and other issues.

The Indian Pharma manufacturers have the advantage of cheaper manufacturing resource costs. Yet, they have been draining a lot of human value and are susceptible to all the FDA compliance issues and losing all the manufacturing productivity. One product recall or FDA warning letter is so expensive for any size of Pharma Manufacturer to overlook as it will wipe out all the margins by running with cheaper human resources and paper-based logbooks.
The Myth has been in the industry that these systems are expensive than human resources, which is not the case anymore. This is what has been witnessed in the last five years with several big Indian pharma companies. What is needed for the industry is the digital solution or a digitization platform that keeps real-time monitoring of all aspects of the manufacturing and ensures close adherence to the compliance standards such as 21 CFR Part 11, MHRA, and cGMP practices. Suppose Indian pharma manufacturing companies move to Atachi systems cloud-based eLogbook solutions. Companies need not worry about Audit and Compliance issues because we built all our NGIMES solution (MES, eLogbook, DMS, QMS) according to 21 CFR Part 11 Compliance (FDA compliance). Atachi eLogbook ensures operational checks, maintenance services, equipment cleaning, calibration, quality checks, and training.
Atachi systems eLogbooks is different from other offerings in the market.
There are other eLogbook offerings in the market; many don’t have 21 CFR Part 11 compliance built-in, and others just provide the electronic data entry. The eLogbook doesn’t mean simply digitizing your paper, not at all. If that’s the case, the internal IT department in any company can give you many forms using any free tools available at their disposal.
What Atachi systems was bring to the customers when they deployed our eLogbooks: Real-time 360 degree monitoring of assets that include equipment, area, process steps meaning adherence to the CGMP standards and transparent control on the quality and many more benefits immediately.
For example, one of our customers leveraged our eLogbooks and data analytics solution and improved their equipment cleaning cycles, and improved the throughput. Another customer moved to the predictive maintenance schedule and reduced a lot of downtimes.
Atachi eLogbooks capture the data and provide actionable insights from the data and improve the operation and business results for our customers. Atachi eLogbook also provides complete analytics for free, so they don’t need to buy any other analytics tools. In the near future, if customers want to capture the data directly from PLC’s and do eBMR, Atachi provides seamless integration since the platform already has these functionalities, a way for Pharma 4.0 journey.
Atachi systems offer a free eLogbook. What does it mean?
Atachi systems is trying to replace the paper-based logbooks with electronic logbooks for all the pharma companies for free. As part of the promotion. Atachi is committing to give the Indian pharma community up to 50 free eLogbooks license, without any strings attached. The intentions are evident if the customers realize the value of these free eLogbooks and when they need help with any other functionalities such as eBMR, QMS & MES/IIOT they might think about us. We extend the same superior customer experience and quality product to all the customers who take advantage of this offer.
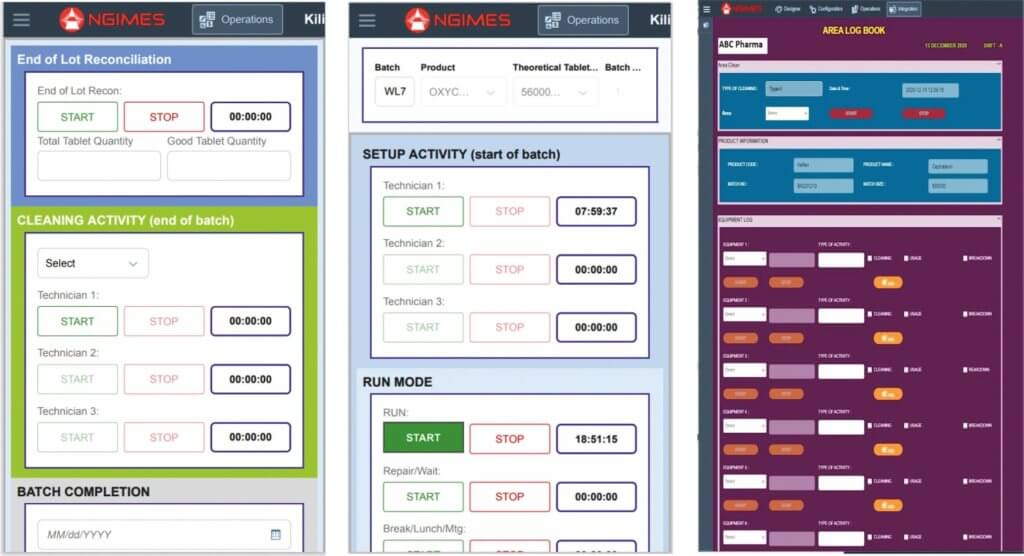
KEY BENEFITS WITH ATACHI’S eLOGBOOKS :
1. Enhanced Compliance: Our eLogbooks are fully cGMP, FDA, MHRA, EMA Compliant.
2. Completely customized: eLogs are completely customized as per your need eg., QA/QC Logs, Area cleaning logs, equipment logs, Sanitation logs, etc.
3. Saves Production Time: Eliminates paper completely, save hundreds of tedious man-hours in generating, reviewing, and approving multiple logs/registers.
4. Complete Traceability: Have complete traceability in audit trial, viz., timestamps, operating personnel, eSignatures, etc.
5. Process excellence: Prevent usage of wrong equipment, wrong workmanship, maintain your process in a controlled & validated state.
6. Real-time reports & Analytics: Log data is clearly depicted via reports in the format you require. Analytics runs on top of it, giving you key insights – Which type of cleaning saves time (Type A or B) , which equipment is to be scheduled for maintenance, etc.
7. Alerts & Deviations: Set alerts & deviations via text messages, emails in real-time.
8. Compatibility: Our eLogs can run on a simple browser via a mobile phone, a galaxy tab – No additional IT server or infrastructure burden. It supports 18+ regional/international languages like Telugu, Hindi, etc.
How we differ from market?
There are other eLogbook offerings in the market; many don’t have 21 CFR Part 11 compliance built-in, and others just provide the electronic data entry. The eLogbook doesn’t mean simply digitizing your paper, not at all. If that’s the case, the internal IT department in any company can give you many forms using any free tools available at their disposal.
What Atachi systems was bring to the customers when they deployed our eLogbooks: Real-time 360 degree monitoring of assets that include equipment, area, process steps meaning adherence to the CGMP standards and transparent control on the quality and many more benefits immediately.
For example, one of our customers leveraged our eLogbooks and data analytics solution and improved their equipment cleaning cycles, and improved the throughput. Another customer moved to the predictive maintenance schedule and reduced a lot of downtimes.
Atachi eLogbooks capture the data and provide actionable insights from the data and improve the operation and business results for our customers. Atachi eLogbook also provides complete analytics for free, so they don’t need to buy any other analytics tools. In the near future, if customers want to capture the data directly from PLC’s and do eBMR, Atachi provides seamless integration since the platform already has these functionalities, a way for Pharma 4.0 journey.
Why do pharma manufacturers need eLogbooks?
eLogbooks(ELN) are considered as a first step to any digital transformation journey. They provide with basic essential data parameters- and this data could be blended with eBMR/QMS/DMS or other modules to make a comprehensive guided data-set to foster strategic business decisions. eLogs eliminates human errors, saves enormous production time – enhancing faster product launches. ELN ensures that robust process control, version control is achieved. Predictive maintenance of equipment, eliminating paper completely, enabling manufacturers to be inline with FDA standards via the digitization of every log data, holistic assessment of area cleaning, sanitation, QA/QC logs, sample management equips every manufacturer out there with vital real-time manufacturing insights ensuring effective operational excellence and manifolded productivity.

